half life formula for zero order reaction
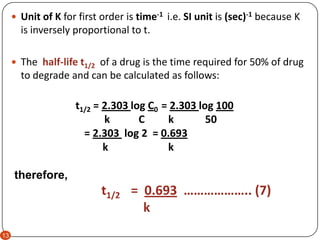
For the first-order reaction the half-life is defined as t 12 0693k. A kt A 0.
Zero Order Reaction Definition Examples Formula
For the 1 st order reaction the half-life is.
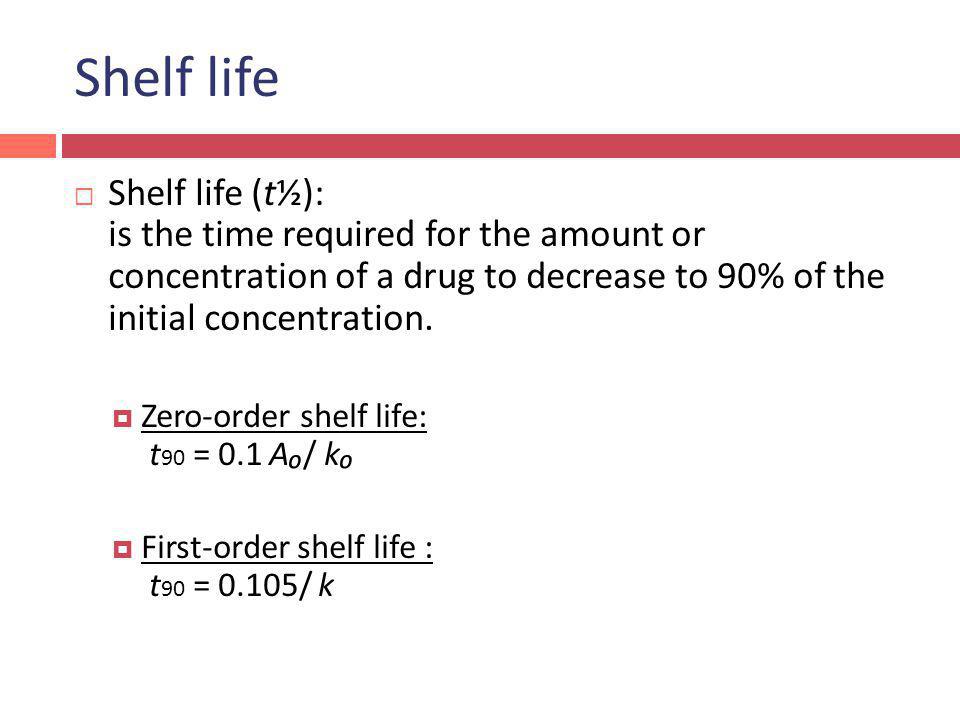
. A A 0 - kt. This is an expression of the half-life of a zero-order reaction. T ½ 1 k A o Top.
Std-12 ChemistryChap-4 Chemical kineticsLecture-09 Zero Order reaction and half life timeIntegrated rate equationPerfect Tuition Classes NeetJeePhysics Wa. T 12 12 k A 0. Half life of Zero order reaction formula is the time at which the initial concentration of reactant becomes half and is represented as T 12 C 0 2 k or Half Life of Zero Order Reaction Initial Concentration for Zero Order Reaction 2 Rate Constant of Zero Order Reaction.
The half-life of a zero-order reaction is given below. For a zero order reaction the formula is t½ Ao 2k. For a general reaction.
Relationship Between Half-life and Zero-order Reactions. A0 A kt. Graphical relations and half lives.
One can describe exponential decay by any of the three formulas. Half-Life for a Zero-Order Reaction The integrated rate law for a zero-order reaction is given by. Half-Life of Zero Order Reaction.
The integrated rate constant for the zero-order reaction is given by. When t t ½ C C o 2 and the equation 87 becomes. It is to be noted that the formula for the half-life of a reaction varies with the order of the reaction.
Half-life is denoted by the symbol t 12. Replacing t with half-life t 12 we get. And for the second-order reaction the formula for the half-life of.
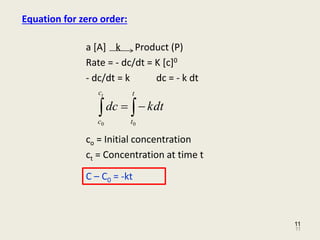
Equation 89 shows that the t ½ of a zero-order process is not constant but proportional to the initial concentration of drug C o and inversely proportional to the zero-order rate constant K o. ½ A kt 12 A 0. Generally the chemical reaction carried out by a chemical catalyst is zero order.
The integrated rate equation for zero-order reactions is the name given to this equation. Where A0 Initial concentration of. We can represent the relationship by the following equation.
The rate constant for a. T_12 is a timescale in which each half-life represents the reduction of the initial population to 50 of its original state. A A 0 k t AA_0-kt A A 0 k t.
Half-life or t½ is the time that elapses before the concentration of a reactant is reduced to half its initial. A A 0 k t. The Half-Life of Zero Order Reaction calculator computes the half-life in nuclear decay for a zero order reaction.
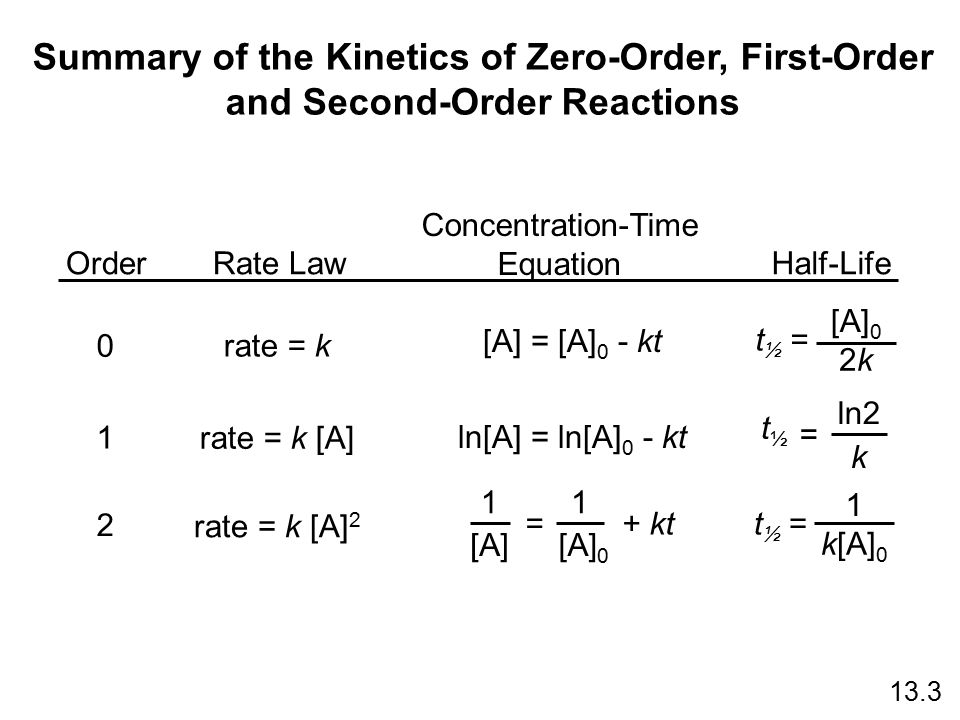
K t 12 12 A 0. N t N0. The half-life of a zero-order reaction the formula is given as t 12 R 02 k The half-life of a first-order reaction is given as t 12 0693k The half-life of a second-order reaction is given by the formula 1kR 0.
What is the half-life equation for a zero order reaction. What is zero order reaction with example. The measurement of this quantity may take place in grams moles number of atoms etc.
The enzyme catalysis reaction is an example of zero order reaction with respect to the substrate. 12 A A 0 - k t 12. T 12 0693k.
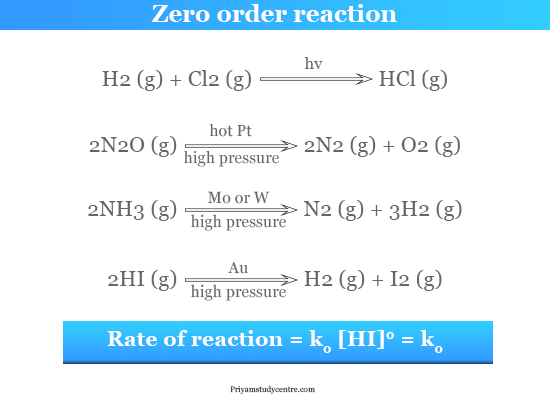
For a zero order reaction A products rate k. Read More Second order Reaction. N t N0.
Determining a half life. From the above-integrated equation we have. Replacing t with half-life t 12 we get.
N t N0. The half-life equation for a zero-order reaction is t12A02k t 1 2 A 0 2 k. The half-life of a Zero-th order reaction is t A0 2kHere I derive this from the Integrated Rate LawAsk me questions.
The half-life of a reaction is defined as the time required for the reactant concentration to fall to one half of its initial value. Equations for Half Lives. Where N0 refers to the initial quantity of the substance that will decay.
T 12 A 0 2k. Half-life t ½ or half-time is defined as the time period required for the concentration of drug to decrease by one-half. Half life formula for Zero order reaction.
The rate law of zero order kinetics is. The mathematical expression that can be employed to determine the half-life for a zero-order reaction is t 12 R 0 2k. Converting a half life to a rate constant.
A Product. Thus for t t 12 A t ½ A o. A zero order reaction implies that the rate of the reaction does not depend on the concentration of the reactant.
This form allows us to calculate the population of the reactant at any point after the reaction has begun. For a first order reaction t½ 0693 k and for a second order reaction t½ 1 k Ao. Do zero order reactions have a half-life.
Now replacing t with half-life t12 in the above equation. It is clearly visible from the above equation that the half-life of the reaction is dependent on the rate constant as well as the initial concentration of the reactant. The formula for half-life in chemistry depends on the order of the reaction.
T he half-life equation for a zero-order reaction is t12A02k t 1 2 A 0 2 k. T 12 is the half-life of the reaction seconds. The timescale in which there is a 50 reduction in the initial population is referred to as half-life.
From the integral form we have the following equation. The Initial concentration for zero order reaction is the concentration of reactant present before the start. T ½ 0693 k For a second order reaction 2A products or A B products when A B rate kA 2.
T ½ A o 2k For a first order reaction A products rate kA. For a zero order reaction Half life decreases with decreasing concentration For a 1st order reaction Half life is constant For a second order reaction.

Chemical Kinetics Integrated Rate Laws Notes

Integrated Rate Laws Zero First Second Order Reactions Chemical Kinetics Youtube
First Order Reaction Derivation And It S Half Life Time Chemical Kinetics Chapter Youtube
Half Life Of A First Order Reaction Video Khan Academy
Derive Half Life For Zero Order And First Order Reaction Chemistry Point

Half Life Expressions Chemistnate

Chapter 14 Chemical Kinetics And Stability Ppt Download
Kinetics Order Of Reactions Ppt Video Online Download

Half Life Of A Zero Order Reaction Is 250sec T75 T100 Of The Reaction Respectively In Sec Are Edurev Neet Question

Derive The Integrated Half Life Equation For Zero Order Reaction Chemistry Chemical Kinetics 12889537 Meritnation Com
Kinetics And Drug Stability Ed

Half Life Of Zero Th 0th Order Reaction Derivation Youtube
Summary Of The Kinetics Of Zero Order First Order Ppt Download

Half Life Expressions Chemistnate

Zero Order Reactions Chemistry Class 12 Iit Jee Main Advanced Neet Aipmt Askiitians Youtube